Class 12 Chemistry Notes on Batteries, Cells and Corrosion
Batteries, Cells and Corrosion
Batteries
These are source of electrical energy which may have one or more cells connected in series.
For a good quality battery it should be reasonably light. compact and its voltage should not vary appreciably during its use.
Primary Batteries
In the primary batteries, the reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again.
(i) Dry cell or Leclanehe cell
Anode-Zinc container
Cathode-Graphite rod surrounded by MnO2 powder
Electrolyte-Paste of NH4Cl + ZnCl2
Cathode reaction,
2MnO2(s) + 2 NH+4(aq) + 2e– → Mn2O3(s) + 2NH3(g) + H2O(l)
Anode reaction,
Zn(s) → Zn2+(aq) + 2e –Cell potential 1.25 V to 1.5 V
(ii) Mercury cell
Anode-Zn-Hg amalgam
Cathode-Paste of (HgO + C)
Electrolyte-Moist paste of KOH-ZnO
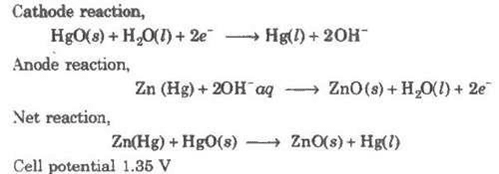
Secondary Batteries
These cells can be recharged and can be used again and again, e.g.,
(i) Lead Storage battery
Anode-Spongy lead
Cathode-Grid of lead packed with PbO2
Electrolyte-38% H2SO4 by mass
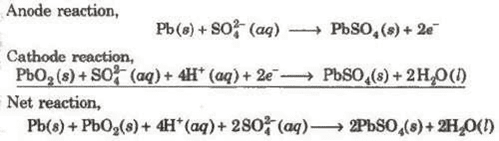
When recharged the cell reactions are reversed.
(ii) Nickel-cadmium storage cell
Anode-Cadmium
Cathode-Metal grid containing NiO2
Electrolyte-KOH solution
Anode reaction,
Cd(s) + 2OH–(aq) → Cd(OH)2(s) + 2e–

Fuel Cells
Galvanic cells which use energy of combustion of fuels like H2, CH4, CH3OH, etc., as the source to produce electrical energy are called fuel cells. The fuel cells are pollution free and have high efficiency.
Hydrogen-Oxygen Fuel Cell
Electrodes-Made of porous graphite impregnated with catalyst (Pt, Ag or a metal oxide).
Electrolyte-Aqueous solution of KOH or NaOH
Oxygen and hydrogen are continuously fed into the cell.
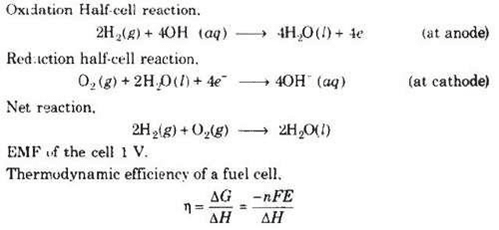
Corrosion
Slow formation of undesirable compounds such as oxides, sulphides or carbonates at the surface of metals by reaction with moisture and other atmospheric gases is known as corrosion.
Factors Affecting Corrosion
- Reactivity of metals
- Presence of moisture and atmospheric gases like CO2, SO2, etc.
- Presence of impurities
- Strains in the metal
- Presence of electrolyte
Rusting of Iron-Electrochemical Theory
An electrochemical cell, also known as corrosion cell, is developed at the surface of iron.
Anode- Pure iron
Cathode-Impure surface
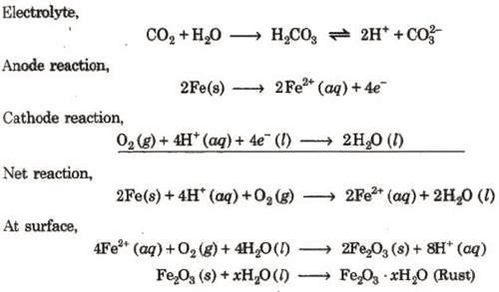
Rusting of iron can be prevented by the following methods :
- Barrier protection through coating of paints or electroplating.
- Through galvanisation or coating of surface with tin metal.
- By the use of antirust solutions (bis phenol).
- By cathodic protection in which a metal is protected from corrosion by connecting it to another metal that is more easily oxidised.