COMPARISON OF SIGMA BOND AND pi BOND
The bond is formed by the overlap of orbitals along their axes (end to end to overlap) . It includes s-s, S-p and p-p of orbitals .
It is a strong bond
Electron cloud is symmetrical about the line joining the two nuclei.
Compounds containing sigma bond are less reactive.
o-bond can have independent
The bond is formed by sidewise overlapping of orbitals (lateral overlapping). It includes p-p overlapping only.
It is a weak bond.
Electron cloud is unsymmetrical.
Compounds containing pi bond are more reactive.
π -bond always exists along with a o-bond.
existence.
What is Pi (π) Bond ?
Pi bonds are formed by the sidewise or lateral overlapping of p-orbitals. The overlapping takes place at the side of the two lobes and hence, the extent of overlapping is relatively smaller. Thus, π -bond is a weaker bond in comparison to sigma bond.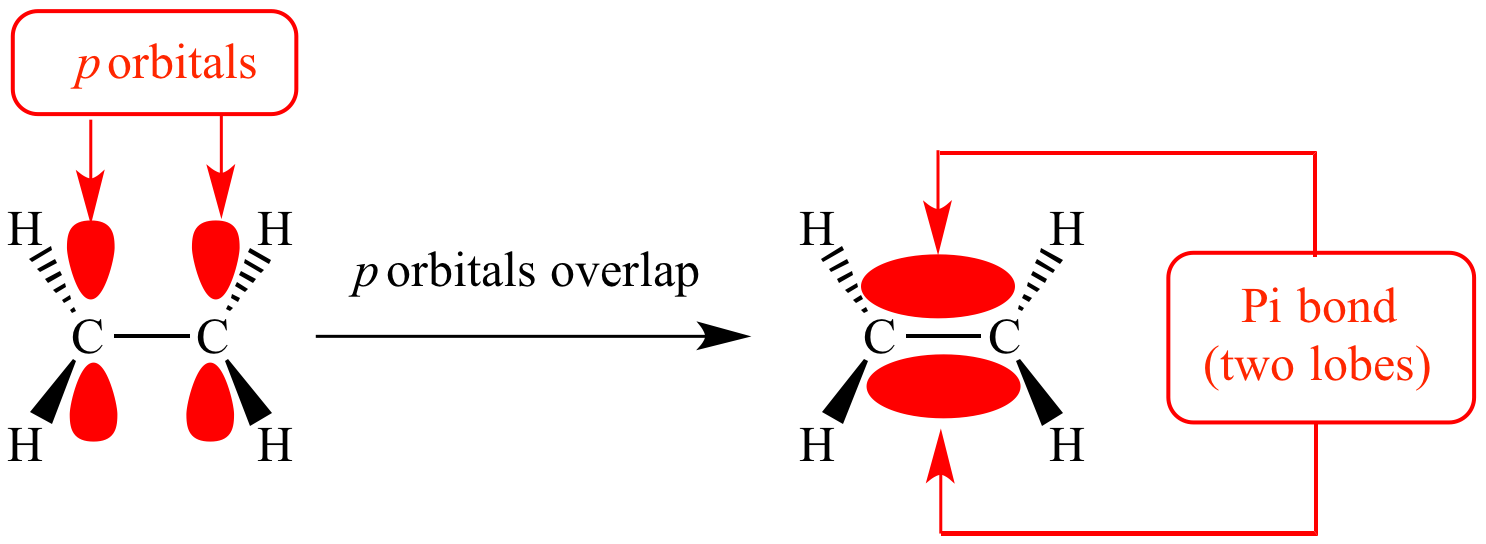
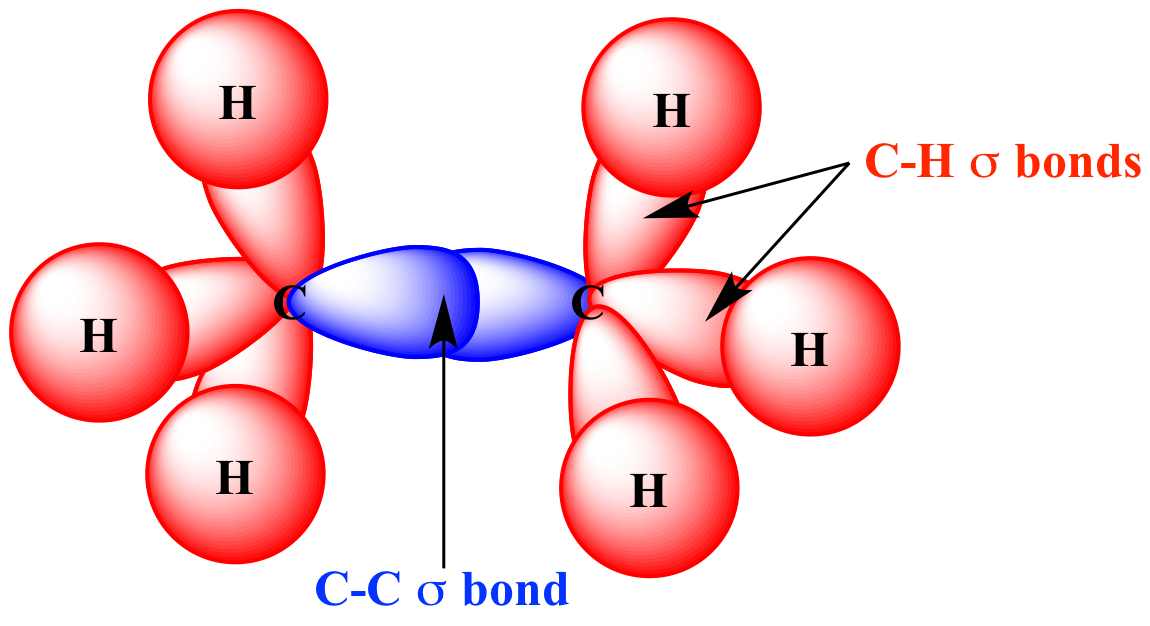
What is Sigma Bond?
Sigma bond :A bond formed between two atoms by the overlap a singly occupied orbitals along their axes (end to end overlap) is called sigma (0) bond. In such a bond formation, maximum overlapping is possible between electron clouds and hence, it is a strong bond. Sigma bond can, thus, be defined as -“Bond orbital which is symmetrical about the line joining the two nuclei is known as sigma bond”.