Are you looking for DAV Books Solutions then you are in right place, we have discussed the solution of Science class 7 book Chapter 4 Acids, Bases and Salts which is followed in all DAV School Solutions are given below with proper Explanation please bookmark our website for further updates!! All the Best !!
DAV Class-8 Acids, Bases and Salts Question and Answer
Something To Know
A. Fill in the blanks.
1. Acids which are present in plants and animals are called ___________.
2. Bases taste ___________ and have a ___________ feel.
3. Acids turns the colour of blue litmus paper to ___________.
4. The products of neutralisation reaction are ___________ and ___________.
5. Salts of nitric acid (HNO3) are named as ___________.
6. Sodium acetate (CH3COONa) is a basic salt formed by the reaction of ___________ and ___________.
Answer: (1) organic acids (2) bitter, soapy (3) red (4) salt, water (5) nitrate (6) acetic acid, sodium hydroxide
B. Match the following.
| Column I | Column II (Answer) |
| 1. Lemon juice | (a) Oxalic acid |
| 2. Tamarind | (b) Lactic acid |
| 3. Vinegar | (c) Citric acid |
| 4. Red ants | (d) Acetic acid |
| 5. Sour milk | (e) Tartaric acid |
| 6. Guava | (f) Formic acid |
C. Tick the correct option.
1. Bases have a-
Ans 1: bitter taste and a soapy feel
2. An example of a natural indicator is-
Ans 2: litmus
3. An acid, that contributes to the sour taste of some fruits, is-
Ans 3: citric acid
4. Which of the following is a strong acid?
Ans 4: nitric acid
5. Substances, produced through a chemical reaction between acids and bases, are known as-
Ans 5: salts
6. An indicator, that turns red in a basic medium, is-
Ans 6. turmeric
7. The general taste, of acids and bases, is respectively-
Ans 7. sour and bitter
D. Answer the following questions in brief.
1. What are mineral acids?
Ans 1: Acids which are prepared from minerals are called mineral acids.
2. Give two examples each of mineral acids and organic acids.
Ans 2: Mineral acids – Sulphuric acid & Nitric acid.
Organic acids – Citric acid & Acetic acid.
3. Name any two substances that can be used as indicators.
Ans 3: Litmus paper and turmeric.
4. Write the meaning of the term ‘neutralisation reaction.
Ans 4: A reaction in which acid reacts with a base to form salt and water is called a neutralisation reaction.
5. Give any two properties of salts.
Ans 5: (1) Salts are formed after the neutralisation reaction.
(2) Salts are bad conductors of electricity in their solid-state but conducts electricity in the water.
6. Classify the following salts as neutral, acidic or basic. Also, write their names.
(a) Na3PO4
(b) K2CO3
(c) NH4NO3
Ans 6: (a) Sodium Phosphate; neutral salts.
(b) Potassium carbonate; basic salts.
(c) Ammonium nitrate; acidic salts.
E. Answer the following questions.
1. All alkalies are bases but all bases are not alkalies. Justify this statement.
Ans 1: Alkalies are the bases which dissolves in water. But all bases cannot dissolve in water. Hence, all alkalies are bases but all bases are not alkalies.
For example- sodium hydroxide is a base, soluble in water and term as an alkali. while aluminium hydroxide is insoluble in water and term as a base not as an alkali.
2. Suggest an activity that can help one to decide whether a given solution is acidic or basic in nature.
Ans 2: Take a solution and a litmus paper. Dip the litmus paper in the solution. If the colour of the litmus paper changes to red then the solution is acidic and when the colour changes to blue then the solution is basic.
3. Write chemical equations for the following reactions:
(a) Calcium hydroxide reacts with nitric acid.
(b) Acetic acid reacts with calcium hydroxide.
(c) Hydrochloric acid reacts with sodium hydroxide.
(d) Ammonium hydroxide reacts with sulphuric acid.
Ans 3:
(a) Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O
(b) 2CH3COOH + Ca(OH)2 → Ca(CH3COO)2 + 2H2O
(c) HCl + NaOH → NaCl + H2O
(d) 2NH4OH + H2SO4 → (NH4)2SO4+ 2H2O
4. State the difference between neutral, acidic and basic salts. Give one example of each.
Ans 4:
Neutral salts
(1) Formed by the reaction of a strong acid and a strong base.
(2) Do not change the colour of litmus paper.
Example: Sodium chloride (NaCl)
Acidic salts
(1) Formed by the reactions of a strong acid and a weak base.
(2) Change the colour of blue litmus to red.
Example: Aluminium chloride (AlCl3)
Basic salts
(1) Formed by the reaction of a weak acid and a strong base.
(2) Change the colour of red litmus to blue.
Example: Sodium acetate (CH3COONa)
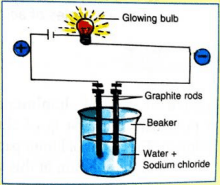
5. Describe an activity to show that solutions of salts, in water, can conduct electricity.
Ans 5: Take a beaker and fill it half with water. Dissolve some common salt (sodium chloride) in this water. Connect two graphite rods with the two terminals of a battery, with a zero-watt LED bulb in between, as shown in the figure. Now dip these graphite rods in the solution of sodium chloride. The bulb starts glowing indicating the flow of electric current. This shows that a solution of sodium chloride can conduct electricity.